
Search

Water purification is the basic utility engineering of pharmaceutical and daily chemical plants, the quality of water will directly affect the quality of the product, therefore, water quality control is the focus of attention of the plant. In addition, the water system is also a major energy consumer in the factory. From the perspective of green economy, how to reduce energy consumption on the basis of ensuring water quality is also a concern of the industry.
The water system consists of a watermaker and a storage and distribution system, and the latter effectively relieves the pressure on the watermaker to cope with peak water usage conditions in the plant.
Typically watermaker systems utilize chemical additives to control microorganisms in the water, while users have different options for microbial control of the storage and distribution system, which is generally sterilized by three technical means of pasteurization, ozonization, and chemical disinfection.
Comparison of common sterilization techniques
Pasteurization: bacteria reproduce more slowly at lower temperatures within a certain temperature range; reproduce more quickly at higher temperatures. But when the temperature gets too high, the bacteria die. Taking advantage of the heat intolerance of pathogens, pasteurization kills bacteria at the right temperature within a reasonable holding time. In water systems, pure water is generally maintained at 65°C-80°C for a period of time to achieve the sterilization purpose.
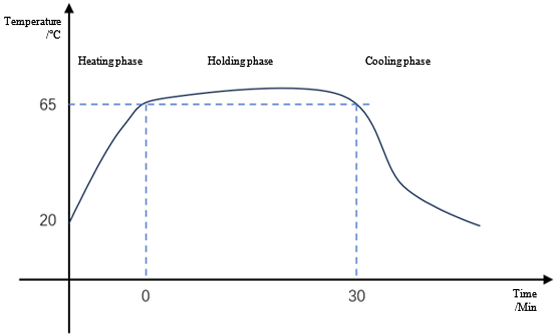
Pasteurization temperature profile in a case
Ozonization: Ozone undergoes redox reaction in water, producing monoatomic oxygen (O) and hydroxyl (OH) with very strong oxidizing power, instantly decomposing organic substances, bacteria and microorganisms in water. Ozone is added to the pure water system and dissolved in the pure water, making use of the oxidizing properties of ozone to continuously sterilize the water in the storage tanks and circulating lines.
Chemical disinfection: Chemical disinfectants act on microorganisms and pathogens, denaturating their proteins so that they lose their normal function and die. After pure water is stored and used at room temperature for a certain period of time, the water system should be shut down for chemical disinfection with commonly used chlorine-containing disinfectants. The system is disinfected after rough washing, cleaning with pharmaceutical solution, rising and other operations.
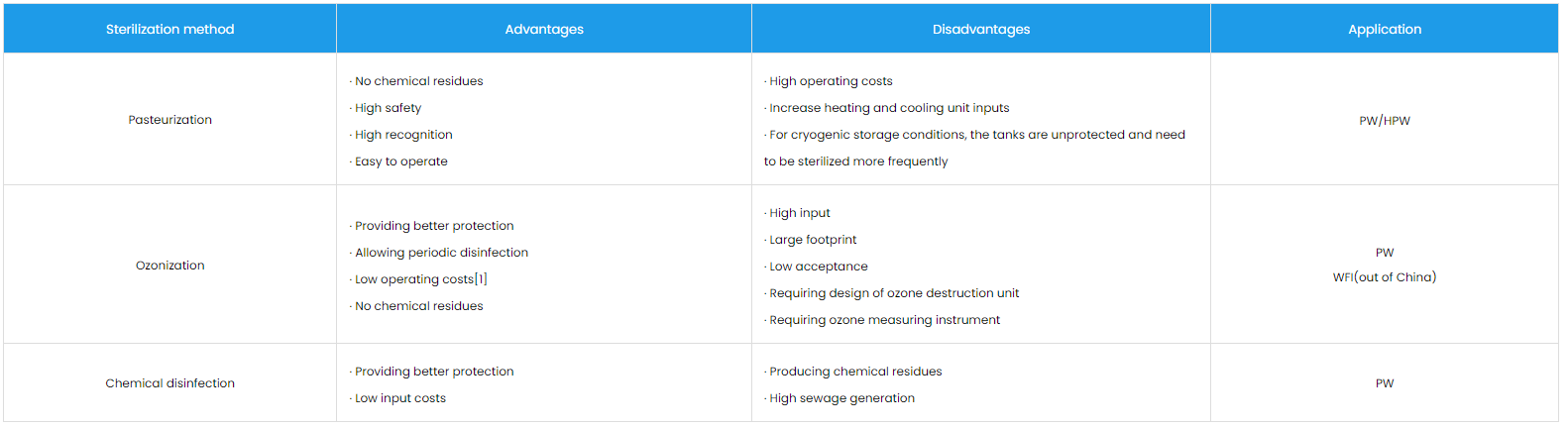
[1] For ozonization, the cost of equipment maintenance also needs to be considered at the time of operation in addition to the need of supporting ozone equipment for construction. Compared with pasteurization, ozonization eliminates the continuous consumption of utilities such as water heating and cooling; compared with chemical disinfection, ozone disinfection eliminates the consumption of large amounts of pure water and the impact of prolonged downtime on plant production.
Besides, ozonization reduces the probability of additional downtime caused by microbial exceedances in the system with its stable and continuous microbial control capability, thus eliminating additional cleaning and disinfection, root cause analysis, modified system validation and other related work. Therefore, ozonization is less expensive to run than the other two methods.
Ozonization technology
Currently there is more than 40 years of foreign experience in the use of ozonization technology in pharmaceutical water systems. The Chinese GMP requires that the storage and distribution of purified water should prevent the growth of microorganisms, and the specific approach is no longer clearly defined. As the technology of the equipment and instrumentation on the market becomes more advanced and users become more aware of it and give it more recognition, the technology will gradually be chosen.
Morimatsu has many cases of ozone technology application in water purification system, which can help customers to ensure the stable and low energy consumption operation of water purification system. In 2021, Morimatsu was awarded the first factory certificate in the industry for complying with German Water Resources Regulations issued by TÜV, contributing to global sustainability.
Next, we will demonstrate ozonization technology from the prospectives of ozonization principle, ozone system composition and safety protection.
Ozonization principle: Ozone is the gaseous three-atomic form of oxygen (O3), an allotropic isomer of oxygen. It can be produced naturally in the atmosphere by irradiating oxygen (O2) with ultraviolet light (185 nm). Ozone is extremely unstable at atmospheric temperatures and pressures, and will be decomposed to free radicals that can continue to undergo oxidation reactions and act as a disinfectant.
Compared to other commonly used chemical disinfectants, such as chlorine, ozone has a very high lethal coefficient. Chlorine must first diffuse into the bacterial cell to cause lethality, while ozone kills bacteria primarily by attacking the organic components of the bacterial cell wall and cell membrane. It may lead to cell rupture or “lysis”, which is a much faster destruction mechanism (up to thousands of times faster) than that of chlorine. In addition, ozone is reduced to oxygen after sterilization, without residue or secondary pollution, so it is known as a green element.
Ozone system composition: Ozonization system is composed of an ozone generation system, an ozone destruction unit and related control devices. Configurations vary slightly between different systems. ISPE describes system design options for applying different ozone production methods, such as air corona, oxygen corona, and water electrolysis.
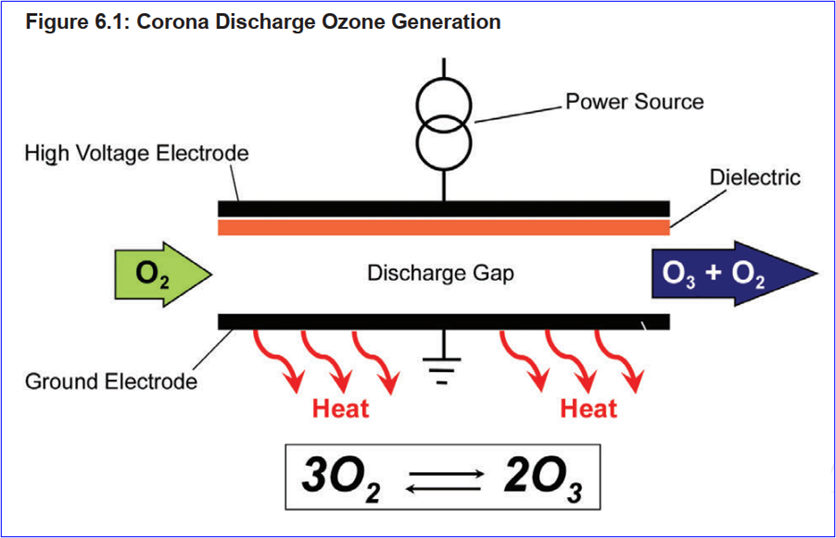
Corona
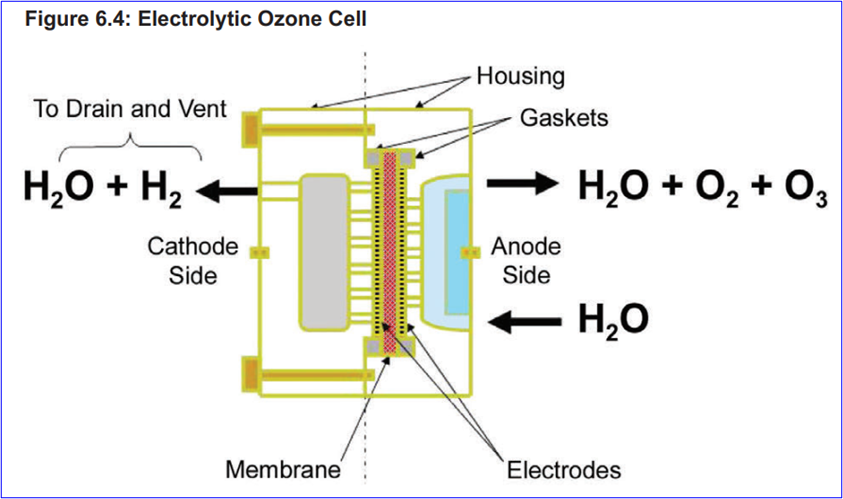
Water electrolysis
The following figure shows an example of a configuration of a disinfection technology suitable for the production of ozone by electrolysis of water:
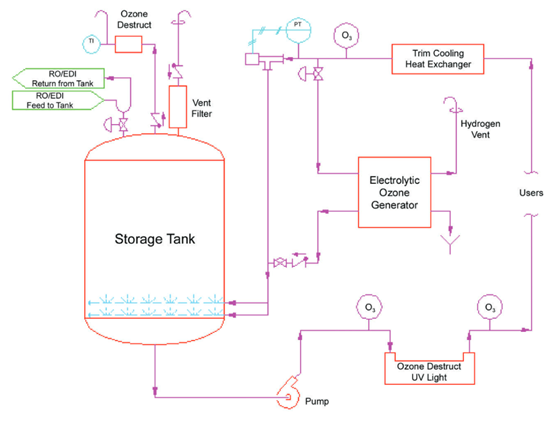
The storage tanks and recirculation loops of the pure water system work at ambient temperature, and use an electrolytic water ozone generator to add ozone to the water. The water in the tank is continuously disinfected by maintaining an effective level of ozone within the tank. As the water enters the recirculation loop, it passes through a UV ozone depletion system where the ozone is destroyed before it reaches the point of use. The recirculation loops and the system should be sanitized periodically, and the ozone concentration in the recirculation loops and the entire system is increased by turning off the UV lamps. Tanks are more prone to microbial growth than recirculation loops, and the dissolution of ozone in the tank water and the presence of ozone gas at the top of the tank can effectively control the microorganisms in the tank.
Ozone allows for continuous microbial control of the system, and cyclically increasing the ozone concentration for overall system disinfection based on sampling data will effectively control microbes for high quality purified water.
Factors affecting system performance include ozone concentration, water temperature, water flow rate, UV levels, and system instrumentation. A good ozonization system cannot be separated from proper equipment, reasonable installation, mutual use, complete operation instruction and equipment maintenance. In general, an ozonization system is used in the pure water system, and users should:
- Control the ozone concentration in tank water or upstream of the UV ozone destruction unit to ensure microbial control in the tank.
- Make sure there is sufficient UV dose to destroy ozone.
- Adjusts the concentration of dissolved ozone in the water after UV destruction to ensure the safe use of pure water.
- Control the water temperature to meet the user’s requirements for water temperature while allowing enough ozone to dissolve.
- Monitor the ozone concentration in the return line to monitor the ozone disinfection of the intermittent pure water loop.
Safety protection: Ozone is an oxidizing agent and it is safe when used properly. If not used properly, ozone may be hazardous to the environment, operators, and equipment. High concentrations of ozone pollution in the environment may irritate our respiratory tract, eyes and skin, causing respiratory diseases and jeopardizing human health. Therefore, the following points should be of concern for ozone safety:
- Tank tops that contain ozone should be emptied of tail gases and should be considered for direct venting or discharge after ozone removal, depending on the regulations of the place of use. A safe location should be selected for discharge so that there will be no impact on personnel and plant air conditioning systems.
- When the line is ozonated, the programmed user point of control will avoid the use of pure water containing ozone. However, for manual point-of-use and sampling points, appropriate SOPs should be implemented to avoid personnel misuse and discharge of pure water containing high concentrations of ozone.
- Although leaks have been checked when the system is put into service, there is no guarantee of new leaks during operation. Therefore, one or more ambient ozone monitors should be adopted to ensure continuous monitoring of ozone levels in the environment and rapid detection of leaks.
Conclusion
Ozonization, as a holistic technology, is a complex system design process that requires consideration of the compatibility of all functional components and the operational logic in order to achieve the desired goal.
Ozonization is an ideal choice for plants that are large users of cold purified water. With good equipment configuration and sufficient use management, ozonization technology allows for effective control of microorganisms and energy consumption reduction in the purified water system, quite safe and reliable.
Knowing the principle and benefits of the ozonization system will help users have a more comprehensive understanding of technical solutions in new or modified systems, and make adequate comparisons and choices. We believe that with the increase of application cases and the accumulation of operation data, ozonization technology will better help the pure water system to reduce energy consumption and operate with high quality.
The article is provided by the Technology Division of Morimatsu LifeSciences
About Morimatsu LifeSciences
Morimatsu LifeSciences is a subsidiary of Morimatsu International Holdings Limited (Morimatsu International, stock code: 2155.HK), covering pharmaceutical, biopharmaceutical, FMCG, cosmetics, electronic chemicals and other industries, provides clients with "core equipment/Machinery + Value added + digitalized intelligence overall Plant Solutions and Service" (" MVP Solutions & Service"), mainly including Shanghai Morimatsu Pharmaceutical Equipment Engineering Co., LTD., Morimatsu (Suzhou) LifeSciences Co., LTD., Shanghai Morimatsu Biotechnology Co., LTD., Shanghai Mori-Biounion Technology Co.,Ltd. and Pharmadule Morimatsu AB., Morimatsu Pharmadule (Singapore) Private Limited as well as their subsidiaries. Morimatsu LifeSciences is focusing on the research and development, manufacturing, and sales of products in related fields.
From the start in Japan, Morimatsu has grown and today is a diversified multinational corporation with vast experience and professional know-how in fields of process engineering equipment as well as modular engineering solutions. Our company has established long-term collaboration relationships with numerous well-known global corporations. We have companies in Sweden, Italy, US, India, Singapore, Malaysia, together with the large scale operation and manufacturing facilities in Asia. So far, Morimatsu has delivered projects to more than 40 countries/regions in the world, established an excellent industrial reputation in the process.
Forward-Looking Statements
The information in this press release may include some forward-looking statements. Such statements are essentially susceptible to considerable risks and uncertainties. The use of "predicted", "believed", "forecast", "planned" and/or other similar words/phrases in all statements related to our company is to indicate that the statements are forward-looking ones. Our Company undertakes no obligation to constantly revise such predicted statements.
Forward-looking statements are based on our Company management's current perspectives, assumptions, expectations, estimations, predictions and understanding of future affairs at the time of the making of such statements. Such statements are not guarantees of future development and are susceptible to the impact of risks, uncertainties and other factors; some are beyond the control of our Company and unpredictable. Subject to the influence of future changes and development in our business, competition environment, political, economic, legal and social conditions, the actual outcomes may differ significantly from the information contained in the forward-looking statements.